An Ultra-Rare Disease with a Severe Prognosis
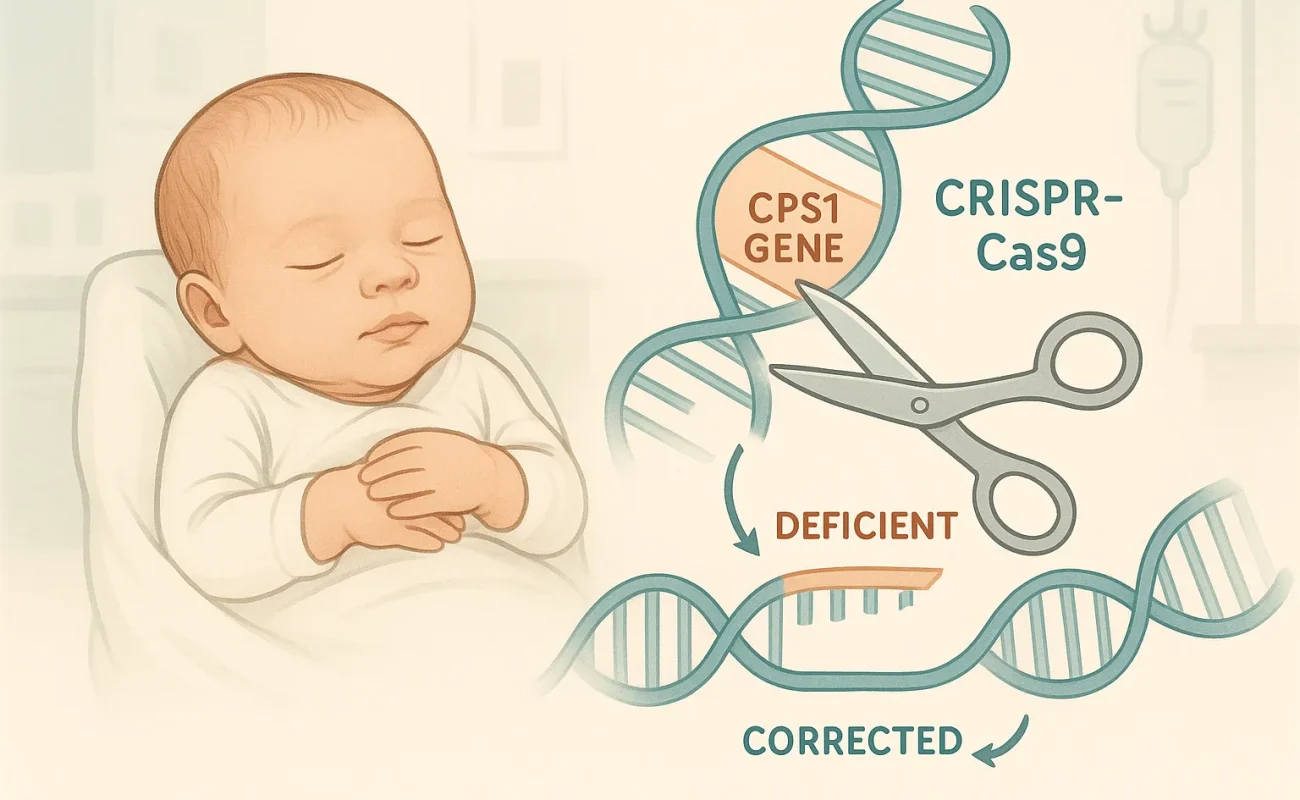
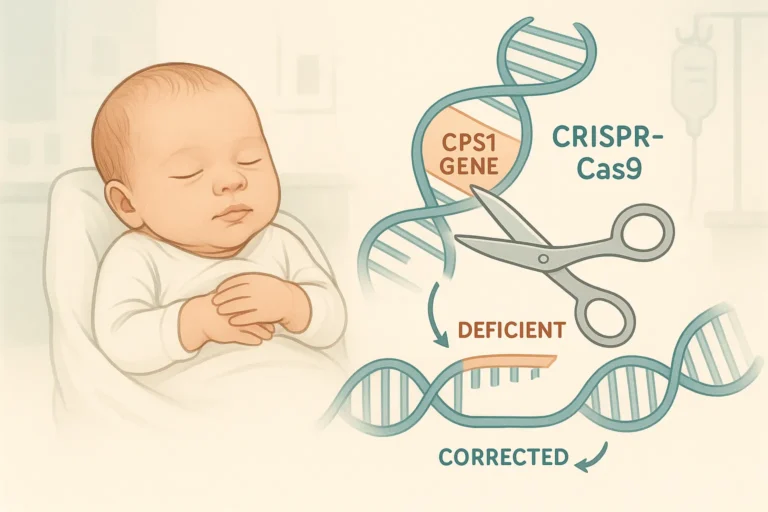
Personalized CRISPR-Cas9 gene therapy addresses carbamoyl phosphate synthetase 1 (CPS1) deficiency, an ultra-rare metabolic disorder impacting about one in 800 000 live births. This condition presents in early infancy with toxic ammonia accumulation (hyperammonemia), often leading to coma, irreversible neurological injury, and 70–80% neonatal mortality without curative intervention. Survivors frequently endure profound neurodevelopmental sequelae.
A Major Breakthrough in Treating a Rare Metabolic Disorder
For the first time, an infant harboring two truncated CPS1 variants (Q335X paternal and E714X maternal) underwent a personalized CRISPR-Cas9 base‐editing therapy. This approach precisely converts the single nucleotide A>G, restoring enzymatic function without inducing double-strand breaks or relying on complex single-stranded oligonucleotides (ssODNs).
Precise Base Editing Strategy
The base editing platform leverages an engineered Cas9 deaminase to mediate the A>G correction, minimizing off-target alterations and enhancing overall safety. By avoiding double-strand DNA breaks, this method reduces p53 activation and genotoxic stress.
Targeted Delivery via Lipid Nanoparticles (LNPs)
Lipid nanoparticles co-encapsulate Cas9 mRNA and liver-specific guide RNAs, enabling:
- Exceptional hepatocyte targeting
- Low immunogenicity
- Safe, repeatable dosing
This tissue-selective delivery ensures efficient genomic correction with minimal systemic exposure.
Rapid Development and Clinical Success
Designed and validated in six months, the customized therapy was administered without serious adverse events. Clinical monitoring demonstrated durable genomic correction and normalization of ammonia levels, offering unprecedented hope for rare metabolic disorders lacking effective treatments.
Toward Agile, Personalized Genomic Medicine
This milestone underscores the maturity of CRISPR-Cas9 base editing combined with advanced delivery systems. It heralds a future of modular, safe gene therapies that can be rapidly tailored to individual patients with rare genetic diseases.
Sources :
NIH News Release – Infant with rare, incurable disease is first to successfully receive personalized gene therapy treatment
https://www.nih.gov/news-events/news-releases/infant-rare-incurable-disease-first-successfully-receive-personalized-gene-therapy-treatment
Children’s Hospital of Philadelphia – World’s First Patient Treated with Personalized CRISPR Gene Editing Therapy
https://www.chop.edu/news/worlds-first-patient-treated-personalized-crispr-gene-editing-therapy-childrens-hospital
Le Monde – Un nourrisson atteint d’une maladie génétique ultra-rare reçoit un traitement personnalisé inédit
https://www.lemonde.fr/realites-biomedicales/article/2025/05/17/un-nourrisson-atteint-d-une-maladie-genetique-ultra-rare-recoit-un-traitement-personnalise-inedit_6606541_6579630.html